From Your Data Is The Ratio Of Metal To Hydrogen Gas 2:1, 1:1 Or 2:3?
Hydrogen Compared with Other Fuels
Similar gasoline or natural gas, hydrogen is a fuel that must be handled properly. It can be used as safely every bit other common fuels when unproblematic guidelines are followed.
Produced by the U.S. Section of Energy, the Hydrogen Data Book. provides useful data on hydrogen properties, including:
- Chemical characteristics of hydrogen (e.thou., density, flammability range, boiling point characteristics, heating values)
- Comparisons of the characteristics of hydrogen and many other fuels.
Bar charts presented in the following sections compare some cardinal properties of hydrogen with those of several ordinarily used fuels - natural gas, propane, and gasoline vapor.
Gaseous Hydrogen
Hydrogen is colorless, odorless, tasteless, not-toxic, and not-poisonous. It's as well non-corrosive, only information technology tin embrittle some metals. Hydrogen is the lightest and smallest chemical element, and information technology is a gas under atmospheric weather.
Natural gas and propane are too odorless, only manufacture adds a sulfur-containing odorant so people can notice them. Currently, odorants are not used with hydrogen considering there are no known odorants light plenty to "travel with" hydrogen at the same dispersion rate. Current odorants besides contaminate fuel cells, which are an important application for hydrogen.
Hydrogen is virtually 57 times lighter than gasoline vapor (as shown in Effigy i) and xiv times lighter than air. This ways that if it is released in an open environment, it will typically rise and disperse apace. This is a safety reward in an exterior surroundings.
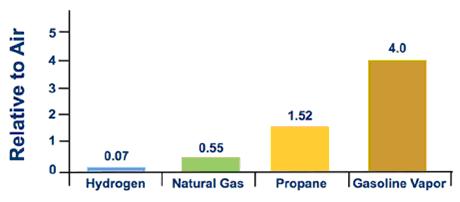
Figure 1. Relative Vapor Density
Hydrogen is a very small-scale molecule with low viscosity, and therefore prone to leakage. In a confined space, leaking hydrogen can accumulate and reach a combustible concentration. Any gas other than oxygen is an asphyxiant in sufficient concentrations. In a closed environs, leaks of any size are a concern, since hydrogen is impossible for human senses to detect and tin ignite over a wide range of concentrations in air, as discussed in the department below. Proper ventilation and the employ of detection sensors can mitigate these hazards.
Hydrogen has a loftier energy content by weight, but not by book, which is a particular challenge for storage. In order to store sufficient quantities of hydrogen gas, it's compressed and stored at loftier pressures. For safety, hydrogen tanks are equipped with pressure level relief devices that will prevent the pressures in the tanks from becoming as well high.
Hydrogen Combustion
The auto-ignition temperature of a substance is the everyman temperature at which it will spontaneously ignite without the presence of a flame or spark. The auto-ignition temperatures of hydrogen and natural gas are very similar. Both take auto-ignition temperatures over 1,000°F, much higher than the machine-ignition temperature of gasoline vapor, as shown in Figure two.
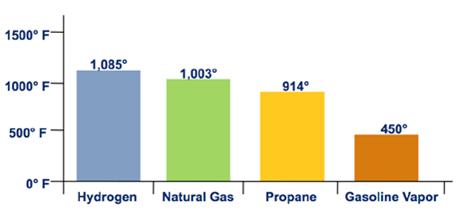
Figure 2. Auto Ignition Temperature
Hydrogen's flammability range (betwixt iv% and 75% in air) is very broad compared to other fuels, every bit shown in Effigy 3. Under the optimal combustion condition (a 29% hydrogen-to-air volume ratio), the energy required to initiate hydrogen combustion is much lower than that required for other common fuels (east.thou., a modest spark will ignite it), every bit shown in Effigy 4. But at low concentrations of hydrogen in air, the free energy required to initiate combustion is similar to that of other fuels.
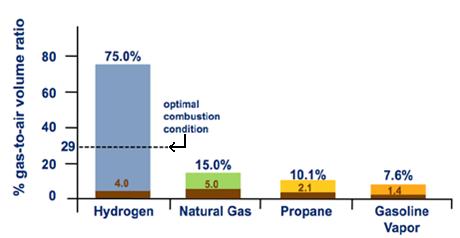
Figure 3. Flammability Range
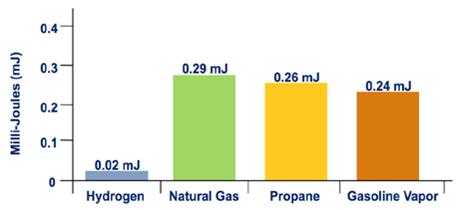
Effigy iv. Minimum Ignition Energy
Hydrogen burns with a pale blue flame that is virtually invisible in daylight, so it is almost impossible to detect by the homo senses (see Hydrogen Flame Characteristics video under Supporting Examples in the right column of this page). Impurities such as sodium from ocean air or other burning materials will innovate color to the hydrogen flame. Detection sensors are about always installed with hydrogen systems to quickly identify any leak and minimize the potential for undetected flames. Compared to the propane flame (right) in Figure 5, the hydrogen flame (left) is nigh invisible, but it can be seen with the thermal imaging camera shown in the foreground. At nighttime, hydrogen flames are visible, as shown in Figure 6.
In addition, hydrogen flames radiate little infrared (IR) heat, just substantial ultraviolet (UV) radiation. This means that when someone is very close to a hydrogen flame, there is little sensation of rut, making inadvertent contact with the flame a significant business. UV overexposure is also a concern, since it can result in sunburn-similar furnishings.
If a large hydrogen cloud comes into contact with an ignition source, ignition will consequence in the flame flashing back to the source of the hydrogen. In open spaces with no confinement, flames will propagate through a flammable hydrogen-air deject at several meters per 2nd, and even more speedily if the cloud is above ambient temperature. The issue is a rapid release of heat, but little overpressure, and the combustion product is steam. Information technology should be noted that hydrogen combustion is more rapid than combustion of other fuels. A hydrogen cloud will fire within seconds, and all of the energy of the deject will be released.
Even so, if hydrogen gas mixtures enter confined regions, ignition is very likely and tin can result in flame acceleration and generation of high pressures capable of exploding buildings and throwing shrapnel. Flammable mixtures of hydrogen in confinements such as pipes or ducts, if ignited, will readily result in accelerated flames and weather condition that can atomic number 82 to transition to detonation. Detonation does not occur in unconfined hydrogen-air mixtures without strong shockwaves (i.east., explosives).
A leak in a pressurized (>200 psia) hydrogen storage system will upshot in a jet that may extend for some meters. If ignited, the jet flame can cause serious damage to annihilation it encounters.
Liquid Hydrogen Expansion
Liquid hydrogen has different characteristics and dissimilar potential hazards than gaseous hydrogen, so different control measures are used to ensure rubber. As a liquid, hydrogen is stored at -423°F, a temperature that can cause cryogenic burns or lung damage. Detection sensors and personal protective equipment are critical when dealing with a potential liquid hydrogen leak or spill.
The volume ratio of liquid to gas is approximately 1:850. And so, if you motion-picture show a gallon of liquid hydrogen, that same amount of hydrogen, existing every bit a gas, would, theoretically, occupy about 850 gallon containers (without compression). Hydrogen undergoes a rapid phase change from liquid to gas, then ventilation and pressure relief devices are congenital into hydrogen systems to ensure safety.
Liquid hydrogen is also colorless. It is extremely cold and just persists if maintained in a cryogenic storage vessel. Storage is usually under pressures upward to 150 psi. If spilled on ambient-temperature surfaces, liquid hydrogen volition apace eddy and its vapors will expand rapidly, increasing 848 times in volume as it warms to room temperatures. If the liquid hydrogen is confined (such as between valves closing off a length of piping) and left to warm without force per unit area relief, pressures budgeted 25,000 psia are possible. With the exception of specially designed enclosures, there is a high potential for exposed confinements to rupture nether such pressures, producing high-pressure jets of gas and high-speed shrapnel. Ignition is extremely probable nether such circumstances. If big quantities of hydrogen displace the oxygen in the air, hydrogen will human activity as an asphyxiant.
Hydrogen Properties Influence Design
An understanding of the properties of hydrogen is critical for the proper design of a facility or workspace. A workspace tin can exist configured to mitigate hazards by understanding and taking advantage of some of the characteristics of hydrogen. Some typical properties of hydrogen, methane, and gasoline are presented in the department Impact of Hydrogen Properties on Facility Design.
From Your Data Is The Ratio Of Metal To Hydrogen Gas 2:1, 1:1 Or 2:3?,
Source: https://h2tools.org/bestpractices/hydrogen-compared-other-fuels
Posted by: hadlockemenceapery2002.blogspot.com


0 Response to "From Your Data Is The Ratio Of Metal To Hydrogen Gas 2:1, 1:1 Or 2:3?"
Post a Comment