How Do Pharmaceutical Companies Get Electronic Medical Data
Introduction
In that location is growing interest in using data captured in electronic health records (EHRs) for patient registries. Both EHRs and patient registries capture and use patient-level clinical information, merely conceptually, they are designed for different purposes. A patient registry is divers as "an organized system that uses observational report methods to collect uniform data (clinical and other) to evaluate specified outcomes for a population divers by a particular disease, condition, or exposure and that serves i or more than predetermined scientific, clinical, or policy purposes."1
An EHR is an electronic system used and maintained by healthcare systems to collect and store patients' medical information.c EHRs are used across clinical intendance and healthcare administration to capture a variety of medical information from individual patients over time, besides as to manage clinical workflows. EHRs comprise different types of patient-level variables, such equally demographics, diagnoses, problem lists, medications, vital signs, and laboratory data. According to the National Academies of Medicine, an EHR has multiple cadre functionalities, including the capture of health data, orders and results direction, clinical decision support, health information exchange, electronic communication, patient support, administrative processes, and population health reporting.ii
In summary, registries are patient-centered, purpose-driven, and designed to derive data on defined exposures and wellness outcome. In contrast, EHRs are visit-centered and transactional. Despite these differences, EHRs capture a wealth of data that is relevant to patient registries. EHRs too may assist in certain functions that a patient registry requires (e.g., information collection, data cleaning, data storage), and a registry may augment the value of the information nerveless in an EHR (e.g., comparative safe, effectiveness and value, population direction, quality reporting).3
EHRs provide a unique opportunity for health systems to develop internal registries or contribute to external registries. Within a health organisation, registries are frequently adult by integrating registry functionalities with existing EHR platforms (i.e., EHR-integrated registries); however, these registries are limited to the health system'southward patient population and may exist unable to capture longitudinal data from dissimilar provider settings. Registries that capture EHR data from multiple health systems typically interface with EHRs to receive data on an interval ground (i.e., EHR-linked or EHR-reported registries), although automating such efforts and creating a bidirectional exchange of information are still challenging.
The Meaningful Use plan (see Chapter ane) has propelled the development of both EHR-linked and EHR-integrated registries. For instance, EHR-integrated registries have expanded to meet EHR certification requirements and to assistance health systems see requirements for workflow efficiency and quality improvement to achieve value-based criteria (e.g., improving population wellness). EHR-linked registries have grown equally the Meaningful Use program specifically requires the reporting of EHR information to external registries (e.g., public health registries, quality reporting registries).four Meaningful Use Stage-i provided an optional objective (which became a mandatory objective in Meaningful Use Stage-2) for eligible hospitals and professionals to submit EHR-extracted electronic data to immunization registries.5 Meaningful Employ Phase-2 further expanded EHR reporting to cancer registries and other specialized registries (e.g., nascence defects, chronic diseases, and traumatic injury registries).6
Driven in big part by Meaningful Utilize, EHR vendors and clinical providers are incentivized to develop processes that would facilitate the design and launch of EHR-based registries in the United states. Yet, despite these incentives, the practice of using EHR-based registries is still relatively immature and, like all evolving research programs, faces many challenges.7
The purpose of this affiliate is to draw the opportunities and challenges related to fully integrating or linking EHRs and patient registries. The chapter reviews common and emerging EHR data types that can be incorporated in registries, provides sample apply cases of integrating EHRs and registries, and proposes a series of hypothetical technical architectures to link or integrate a registry with an EHR. The chapter closes with a discussion of possible future directions for EHR-registry integration. Key questions to consider when planning to incorporate data from EHRs as well equally other sources are provided in Appendix B.
Mutual and Emerging EHR Data Types
EHRs provide various types of data that tin can exist linked, integrated, or merged directly into a registry. The Meaningful Use programme has led to the collection of a Common Clinical Data Set (CCDS) across about providers. These data are now generally bachelor in EHRs; the information that are commonly available will likely proceed to expand as Office of the National Coordinator, nether the 21st Century Cures Act, moves toward building Core Data for Interoperability (USCDI) requirement.8 EHRs can as well provide data types of emerging involvement to registries. Both types are described in Tables 4-ane and 4-ii.
Tabular array 4-ane
Mutual data types of EHRs that tin can be integrated/interfaced with internal/external registries.
Table iv-2
Emerging data types of EHRs that can be integrated/interfaced with internal/external registries.
In add-on to these data, EHRs capture a considerable amount of unstructured data (e.g., clinical notes) that can be further candy to excerpt specific data of importance to a registry (due east.m., specific information extracted from radiology reports to decide eligibility).
Information types commonly extracted from EHRs and imported into registries are patient identifiers, demographics, diagnoses, medications, procedures, laboratory results, vital signs, and utilization events. These are discussed further below.
Patient Identifiers
EHRs are designed to facilitate the identification of private patients in clinical workflows. Patient identifiers include patient's total name, date of birth, contact information such as address and phone numbers, name and contact data of the side by side of kin, emergency contact data, and other personal information accounted necessary for healthcare delivery operations (eastward.thousand., employer information, insurance data). For internal operations, EHRs generate a unique patient ID (i.due east., medical tape number) that is used within the care setting to identity a specific patient. Organizations that provide care at multiple facilities (e.grand., a health system with multiple hospitals and outpatient facilities) often have a second patient identifier that can be used to find a patient across the unabridged health network (i.e., master patient record). If a health organisation is connected to a statewide or regional wellness information exchange (HIE), the EHR may include a third patient identifier that has been issued by the HIE (i.due east., statewide master patient index).9
Provisional to receiving proper consents and adhering to Health Insurance Portability and Accountability Act (HIPAA) policies,x patient identifiers stored in EHRs can be used to merge patient EHR records with a patient registry. For example, a registry may collaborate with a statewide HIE to locate the master patient indexes of all registry patients and and then ask multiple providers to locate the EHR records of those individuals using the HIE-issued patient master indexes. However, many registries do non have the option of acquiring main patient indexes from an HIE. These registries typically apply alternative methods for matching patient identifiers and importing EHR data. Potential mistakes in matching registry patients with EHR patients may atomic number 82 to quality problems such equally incomplete or inaccurate data.
Demographics
EHRs generally contain patient demographic information such as age, gender, and ethnicity/race. These data are needed for clinical operations and are mandated by the Meaningful Utilize objectives. The quality of data on age and gender is ofttimes adequate because of the diverse mandates to collect them accurately.11 – 13 Nevertheless, the quality of demographics information may exist afflicted by other factors including mode of measurement, user mistakes, and information conversion issues.xiv EHRs often have a moderate to high missing data charge per unit for non-essential demographic data such as income, marital status, educational activity, employment status, and nationality.15 , 16
Coding standards for demographic information take been published but are non always used. Demographic data such equally education and nationality are often not coded in a standardized arroyo. Age data are governed by HIPAA and accept sharing limitations if they contain a certain level of granularity (e.g., age represented by the exact date of birth or if ages above a certain limit).17 Demographic data are oftentimes used past registries to lucifer patient records across data sources. Thus, legal limitations to sharing demographic data may hinder the development of multi-source/multi-site EHR-based registries that crave demographic data for these purposes.
Diagnoses
Diagnosis often is a primal variable to evaluate a patient for inclusion in a registry. The quality of diagnosis data is frequently acceptable, in part due to various mandates to collect these information accurately.10 – 12 EHRs also include problem lists as a way to capture active versus non-active diagnoses, but the quality of data found in problem lists may need further validation.
Some established vocabulary standards are available to encode diagnosis data. These include the International Classification of Diseases (ICD),18 International Classification of Principal Care (ICPC),19 Systematized Nomenclature of Medicine (SNOMED),xx Diagnostic and Statistical Manual of Mental Disorders (DSM),21 and Read Codes.22 In the U.S., ICD is the near normally used system to capture diagnostic data in both EHRs and registries. Mapping diagnostic data from one coding arrangement to some other is challenging; fifty-fifty mapping diagnoses from one version of a coding system to some other version is difficult (e.g., mapping ICD-ix to ICD-x). In improver, certain diagnostic codes – such equally HIV status and mental affliction diagnoses – are protected by diverse federal and state-level laws23 that may limit the ability to excerpt these codes for apply in external registries.
Medications
In addition to diagnosis, registries ofttimes utilize medication data as eligibility criteria. Many registries too capture medication data to study treatment effect and/or safety. EHRs contain information on prescriptions that are written, while pharmacy claims data contain information on prescriptions that were filled. When EHR medication data are coupled with chemist's shop claims information, a number of important constructs, such as medication adherence and reconciliation rates (eastward.g., medication regimen complexity index)24 can exist derived and reported to a registry.
The quality of EHR medication data is often acceptable due to various mandates to collect medication data in EHRs. Common vocabulary standards for medications include National Drug Codes (NDCs),25 RxNorm,26 Systematized Classification of Medicine's (SNOMED)20 Chemic axis, Anatomical Therapeutic Chemical Classification System (ATC),27 and a number of commercial drug codes such as MediSpan®, Multum®, Generic Production Identifier® (GPI), and First Databank® (FDB). Each coding standard addresses different aspects of a medication (due east.g. drug class, ingredients, dosage).
Potential semantic interoperability issues may ascend when medication data are combined from multiple sources and mapped from one coding system to some other. For example, an RxNorm code (drug class) may map to multiple NDC codes (packaged drug). Furthermore, some EHR-derived medication data may not exist specific enough for research purposes (due east.g., data on generics, like biosimilars, by and large do not reflect which generic product was supplied to the patient).
Procedures
Process data include clinical procedures such as surgery, radiology, pathology, and laboratory. Procedure data can be extracted directly from EHRs and reported to registries; however, procedures reported from one EHR generally only include those procedures taking place within the premises of a provider using the aforementioned EHR and may non include procedures that occurred elsewhere.
Vocabulary standards for procedures include International Classification of Diseases' Clinical Modification (ICD-CM),18 Current Procedural Terminology (CPT)28 and Healthcare Common Procedure Coding System (HCPCS).29 Each coding organisation is designed to capture procedures inside a specific clinical context (e.thou., primary care, hospital facility). EHR-based process data may not have the level of particular necessary for a registry (due east.g., techniques used in a clinical procedure such as a surgical process). These procedure nuances are often entered as unstructured data that usually do not accompany structured EHR-extracts for registries.
Laboratory Data
Currently, the all-time sources of laboratory data are the information systems used by standalone laboratories, which are frequently but non ever incorporated into the EHR. Laboratory data include both lab orders and lab results. Coding standards for lab orders and lab results include the Logical Ascertainment Identifiers Names and Codes (LOINC),xxx the Systematized Nomenclature of Medicine (SNOMED),20 and the Current Procedural Terminology (CPT).28 Currently, there are no mandated laboratory coding system for certified EHRs, and the majority of healthcare providers rely on local coding systems for lab orders/results. This limits the interoperability of multi-site EHR-derived lab data for registries.
In addition, different healthcare facilities may apply different laboratory tests to measure the same analyte, each of which has a different laboratory code. Discussion is needed beyond the provider network on how to link lab items, preferably using automated tools and not a manual process, and so that a single query beyond the network will return all the desired data from multiple EHRs for a single registry. In add-on, certain lab results are protected by federal and state laws (e.g., lab tests revealing HIV condition) and thus might be missing from EHR-extracts reporting to external registries. Further, some laboratory data are accessible to clinicians without incorporation into the EHR; in fact, some lab data require agile steps by the clinician to import into the EHR. Inaccurate interpretations may be fabricated without understanding why some lab information are missing from an EHR.
Vital Signs
EHRs are a primary source of vital sign data. Vital sign data include physiological variables such as height, weight, body mass index, pulse rate, blood pressure, respiratory rate, and temperature. LOINC is the common coding standard for vital signs. Most provider arrangement, however, practise not actively use LOINC codes to capture vital signs in their EHRs equally information technology is not mandated by the Meaningful Use program.
The abyss of EHR-derived vital signs such as tiptop and weight is ofttimes adequate for use in registries. Bug with human errors and units of measurement may bear upon data quality; thus, data cleaning is essential before apply for registries.31 For example, weight and height data may include incorrect units (e.g., pounds reported as kilograms). EHR also may lack proper meta-data that are of import for the clinical interpretation of the data (e.g., sitting versus continuing blood pressure measurements).
Utilization/Cost
Utilization information can exist extracted from EHRs especially when insurance claims data are non available. Note that EHR-level utilization data are limited to events that have occurred within a particular provider'southward facilities and oftentimes exercise non comprise utilization data from other providers. Utilization tin can be divers as toll, hospitalization, readmission, emergency room admission or other meaning healthcare events. The quality and completeness of utilization data are often acceptable due to reimbursement guidelines.12
There are no specific standard utilization coding terminologies for EHRs; nonetheless, most EHRs attach to the utilization guidelines of claims submission policies. A number of reimbursement policies recommend specific reference-coding systems to encode utilization events. Certain utilization events are protected by diverse federal and state-level laws (e.m., mental wellness visit), and a registry may not receive utilization data related to those conditions from an EHR.
Surveys
Survey data are commonly nerveless from self-reported questionnaires; however, clinical data captured by surveys are increasingly stored within EHRs for various purposes. Some EHRs provide standardized surveys that can be accessed via patient portals to capture patient reported outcomes or symptoms (i.due east., Patient-Reported Outcomes Measurement Data Arrangement or PROMIS).32 Take a chance factors and self-reported behaviors often are of import to registries, and such information can be derived from EHR-integrated surveys (e.1000., smoking status, socioeconomic status, housing condition). Besides, registries may add and integrate their own customized questionnaires in EHRs and then that patients can directly enter the necessary data needed for a registry (e.chiliad., make up one's mind eligibility; collect additional information for a written report).
EHR-integrated surveys are prone to sampling, selection, response, and social-desirability biases. The quality of EHR-integrated survey data varies considerably depending on the questionnaire, and the validity and reliability of custom-built EHR-integrated surveys are frequently hard to mensurate in the context of a clinical practice.
Surveys comprehend variable domains and often do not adhere to coding standards. Indeed, surveys measuring the same concept may code their variables differently. One arroyo to reduce bias and fault in survey-nerveless EHR data is to use standardized questionnaires beyond EHRs and healthcare providers. Some of the many standardized questionnaires include the Patient Reported Outcomes Measures (PROMs), Patient Health Questionnaires (PHQ), Health Gamble Assessments (HRA), Life Consequence Checklist (LEC), and Generalized Anxiety Disorder (GAD) screening tools.
Social Data
Social data include variables ranging from individual-level factors to community-level elements (e.1000., smoking status, socio-economic status, housing condition). Social variables are often considered important factors in registries equally these variables enable researchers to understand the underlying social context and potential disparities associated with the upshot of involvement. As an example, social data captured inside a registry can exist used to assess treatment affordability or empathise heterogeneity of treatment effects. Although increasingly recognized as important variables, social and behavioral data are not routinely captured in EHRs.xiv EHR-derived social data are frequently incomplete and limited to a few information types.33 Moreover, social determinants of health that could be imported from data sources such as social services organizations are unremarkably missing in EHRs and registries due to the lack of interoperability.34
Although a number of coding standards have been proposed to standardize social data, almost EHRs apply proprietary coding vocabularies. Social information are ofttimes of low quality, mainly due to incomplete survey responses and the subjective nature of many social questions. Although nigh social data are non subject to HIPAA, they can nevertheless be discipline to other privacy rules such as the Family Education Rights and Privacy Deed (FERPA).35 Establishing linkages amongst patient-level EHR records, social service records, and registries has faced both technical and regulatory challenges in the past.
Patient-Generated Data
Patient-generated data tin include a broad assortment of variables (e.m., physical activity, sleep patterns, cocky-reported sign and symptoms, uploaded claret sugar levels) and may be captured within an EHR through various means (e.1000., integrated personal health records, mobile-health substitution platforms, wearable device interfaces).36 EHR-based patient-generated information are highly customized and inconsistent across EHRs. Standards are condign more available for mobile health and wearables devices,37 but have not all the same been widely adopted for patient-generated information captured within EHRs. Although the quality of the data nerveless by mobile health and wearable devices is improving, accuracy and comparability are still challenging when such data are collected using different devices. Self-entered information collected via surveys (east.g., entering concrete activity types) are bailiwick to a diverseness of choice factors and errors (east.one thousand., overestimating remember of time spent exercising). Information interoperability may become more than challenging as more non-standardized devices enter the market. Additionally, consenting processes via internet and mobile health solutions may be complex, and the creation of large EHR-integrated registries using patient-generated data requires conscientious attention to legal and regulatory issues.38 , 39
Sample Use Cases and Compages of EHR-Based Registries
Registries that comprise EHR information may use a diverseness of IT system architectures. Registry architects must consider the number of participating sites (single-site or multi-site), variety of underlying EHRs (one enterprise-level EHR, multiple EHR installations of the aforementioned vendor, multiple EHRs from different vendors), existence and connectivity to Health Information Exchanges (HIEs) (centralized, federated or distributed), and other factors that affect interoperability.
Following are examples of three "hypothetical" EHR-based registry types, each with a different combination of stakeholders and IT infrastructures (Table 4-2). Registries designed to support clinical intendance are oft based on unmarried enterprise-level EHRs, while registries designed for research are often hosted external to EHRs merely may receive EHR extracts from multiple sources. Public wellness registries, similar to registries designed for research, are often hosted by wellness departments exterior of a single EHR surroundings merely receive EHR reports on a regular basis. Notation, these are generalized examples; actual IT infrastructure and features may vary.
Tabular array 4-three
IT infrastructure and other features of sample registry types using EHR information.
In a fully interoperable ecosystem, registry-specific functionality could be presented in a software-as-a-service or middleware model, interacting with the EHR as the presentation layer on one end and the registry database on the other.3 In this platonic model, the EHR is a gateway to multiple registries and clinical research activities through an open architecture that leverages best-in-class functionality and connectivity. Full interoperability would enable registries to collaborate across multiple EHRs, and EHRs to interact with multiple registries. Comprehensive interoperability, however, has not notwithstanding been realized, and customized It architectures are required to facilitate the integration and interfacing of EHRs with registries.iii The following are examples of It architectures that could support EHR-integrated/linked registries for clinical operations, enquiry projects, and public health missions.
EHR-Integrated Registries To Support Clinical Care
Healthcare providers often develop and manage EHR-based registries that are used to back up clinical care and meet operational goals (referred to hither as 'clinical registries'). To develop clinical registries, providers typically use EHR-based tools that are adult by EHR vendors. These EHR-based registries can facilitate clinical workflow, monitor quality metrics, enable disease/cohort management, and offer population health management features. In item, the Triple Aim of intendance, health and cost has provided a framework to attain value-based intendance while reducing price.40 This framework promotes 'population wellness' while enhancing the individual's experience of care and lowering cost.41 Constructive population health management is essential to ensuring that resources are directed towards improving health outcomes of patients at the highest take chances for developing undesired outcomes. The notion of population health direction necessitated that health providers develop EHR-based registries to focus on high-risk subpopulations (e.g., patients at loftier risk for bloodshed and morbidity, price, hospital and emergency room admission or who have a chronic condition that requires direct direction, such as diabetes).42 , 43
A major challenge with EHR-integrated clinical registries is the lack of out-of-network data in a health network'due south EHR.44 In other words, data generated during patient encounters with out-of-network providers, who may not be using the same EHR, will be missed in the registry resulting in incomplete and sometimes outdated data. Individual health networks oft complement their EHR data with insurance claims to generate a more complete picture of a patient'due south wellness status; however, use of insurance claims is not e'er practical given that a large patient population of a health commitment network may use dozens, if not hundreds, of different insurers. Many challenges of EHR-based population health registries are derived from the overarching challenges within the broader domain of population health information science.45
Clinical registries usually use a centralized architecture and often have an EHR data warehouse as their backbone forth with multiple data marts containing various registry data. The centralized architecture accumulates and manages data in a single and centralized repository. The advantages of a centralized model are: simplicity and efficiency; greater data consistency; and easier patient linkage if the aforementioned patient identifiers are used across the healthcare network. Potential disadvantages of a centralized model include: data capture that is limited to users of a single EHR vendor across the healthcare network (e.g., trouble with integrating a different EHR vendor if a new facility joins the network); and difficult information exchange with registries developed by other networks due to a lack of interoperability.
Healthcare networks often develop clinical registries based on their underlying enterprise-wide EHR compages (Figure four-1). Data collected at dissimilar facilities of a healthcare delivery network (e.g., hospitals and outpatient clinics) are aggregated in a common information repository such every bit an EHR's data warehouse. Facilities not using the same EHR platform face extra work to harmonize and standardize their information before feeding it into the data warehouse. Data warehouses can be used to develop multiple data marts feeding into various registries for different purposes such as quality measures, affliction management, population health management, and public health reporting. Internal clinical registries are sometimes linked to external registries for reporting purposes (eastward.g., PQRS reporting),46 although interoperability challenges may limit such exchanges.
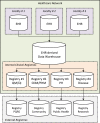
Figure iv-1
Common architecture of EHR-integrated registries to back up clinical care. CDM = Chronic Affliction Direction; HER = Electronic Health Record; PH = Public Health; PHM = Population Health Direction; PQRS = Physician Quality Reporting System; QI = Quality (more...)
EHR-Linked Registries Designed for Enquiry
Registries designed for research purposes (referred to here as 'research registries') may use EHR data on a multifariousness of levels. At the low end, research registries may use EHR data to identify and enroll eligible patients into studies that use supplementary registry-specific data collection. In this scenario, EHR data are used to identify eligible patients (based on the registry's inclusion and exclusion criteria), and minimal EHR data (e.one thousand., family history of breast cancer) are imported into the registry. The remaining registry-specific data are captured through another means, usually a dedicated information repository that allows for entry of eCRFs and web-based survey forms. On the other terminate of the spectrum, some research registries have been built entirely using EHR information (eastward.g., California Cancer registry).47 Many other research registries use a combination of self-reported and EHR data (e.k., Autism treatment network).47 Registries in which EHR-based extracts are merged with registry data on a periodic basis are referred to here as EHR-linked registries.
The increasing semantic and syntactic interoperability among healthcare providers is a major driver for EHR-linked registries. EHR-linked enquiry registries frequently utilise application programing interfaces hosted by healthcare providers to excerpt and share standardized EHR data so use semi-automated approaches to merge the EHR information with existing registry records. Moreover, bi-directionally interoperable EHR-linked registries may too serve an important office by delivering relevant information from a registry back to a clinician (e.g., natural history of affliction, safety, effectiveness, and quality).
EHR-linked research registries collect EHR data using a diversity of mechanisms, ranging from automatic EHR-embedded push protocols to manual advertising-hoc EHR-database pulls. Triggers for EHR data extraction include standardized protocols that follow the inclusion and exclusion criteria of the inquiry registry (i.e., phenotyping queries; retrieve protocols). After receiving the EHR data, research registries use a multi-phase process to import incoming EHR data (Figure 4-ii). Extract, transform, and load functions may include information curation activities such as data preparation, data standardization, secure data transfer, data mapping, data redaction, information integration/merging, and data reconciliation. Various organizations such equally the Clinical Information Interchange Standards Consortium (CDISC) and the Standards and Interoperability (Southward&I) Framework accept introduced detailed mechanisms to automate and standardize the incorporation of EHR information for other purposes including registries (e.k., CDISC Link Initiative48). Additionally, the growing number of common data models have enabled registry developers to attach to specific predefined standards that facilitate integration of EHR-based information as well as data sharing amid registries (eastward.g., Clinical Information Modeling Initiative'due south (CIMI) Reference Model,49 FDA Sentinel Initiative,50 and Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM)).51 Chapter five describes common data models in more detail.
Importing and merging data from EHRs into research registries is challenging. Automating the data imports requires high degrees of interoperability, data curation, and post-hoc harmonization too as attention to data quality. For example, if inclusion criteria are encoded differently in different EHRs, the comparability of data may exist impacted, creating artificial baloney between outcomes measured past unlike EHRs.52 Merging EHR data-imports with existing patient information in a registry also requires reliable primary patient indexing to avoid inaccurate patient-matching which would compromise whatsoever inferences fatigued from the data.ix , 53 Data curation is critical, equally integration of EHR data can expose data quality issues that may affect research findings.54
Information governance must be considered also. Registries designed for inquiry may be funded and managed past a broad range of organizations (e.g., federal, state, not-turn a profit, private). Although patient privacy is safeguarded and protected under federal and state laws,55 data governance policies vary, resulting in unlike barriers for unlike registries when importing and integrating EHR data.56 Additionally, the incentives and liabilities associated with extracting and pushing data from an EHR to an internal or external registry are not always articulate for healthcare providers.57

Figure 4-2
Mutual architecture of EHR-linked research registries. HER = Electronic Health Tape; ETL = Consign, Transform, and Load
EHR-Linked Public Health Registries
Public health agencies have long used registries for surveillance and tracking purposes. For case, local and state public wellness departments usually maintain immunization registries that receive information from clinicians and other entities such as schools and pharmacies. Other common public health registries include syndromic surveillance and specialized registries such equally nascency defects, chronic diseases, and traumatic injury registries. In recent years, coincident with the ascent EHR adoption amidst providers, public health entities began to link diverse registries with EHRs. A significant commuter of increased EHR integration has been the Meaningful Use program, which incentivized clinicians to share EHR immunization and syndromic surveillance data with public health agencies.7 Other drivers take included the maturation of data standards (both semantic and syntactic) for automating and improving the manual of EHR information to public wellness registries (east.one thousand., distributed population queries),58 and the increased interest of value-based care provider organizations in assessing the needs and improving the health of the communities they serve (e.g., community health needs assessment).59 Virtually EHR-linked public wellness registries have relied on semi-automated processes; just recently have more than automated mechanisms been introduced and adopted (e.g., vaccination registries). EHR-linked public wellness registries follow a similar architecture to that of EHR-linked enquiry registries (Effigy four-2); nevertheless, the methods used to collect data from EHRs may vary as not all public wellness registries require patient-level information (e.g., counts are sufficient for some purposes). Methods used include simply are not express to: (1) semi-automated forms/templates to collect public health specific data about patients that fit a certain criteria (e.g., Due south&I Framework SDC);60 (2) data exchange protocols for receiving example reports from certified EHRs (e.g., MU public health reporting objectives);vii (three) tools to mine EHR and HIE data for signs and symptoms relevant to public health emergencies and outbreaks (e.g., ESSENCE Syndromic Surveillance Organisation);61 and, (4) distributed data network queries to collect aggregated information from multiple providers when the identity of patients is non relevant (due east.g., PopMedNet).62
Some public health agencies have been able to direct integrate their registries with the EHRs of clinicians who provide care in their jurisdiction. The prime example of such a fully-integrated EHR-linked public wellness registry is the New York City (NYC) Population Health Registry.63 This registry collects information from NYC's eligible healthcare professionals across several domains (due east.g., Influenza-like-Illnesses). The NYC's Population Health Registry has been successful as near eligible professionals in NYC apply the same EHR system, one which is capable of reporting data in real-fourth dimension to local public wellness agencies. The Population Wellness Registry is function of NYC Macroscope Hub,64 a surveillance system for tracking conditions managed past primary care practices (due east.g., obesity, diabetes, hypertension, and smoking).
Technical Issues and Operational Challenges of EHR-Based Registries
EHR-based registries fulfill different purposes and use unlike IT system architectures, but many technical issues and operational challenges are mutual beyond the range of registries. This department describes several common challenges, such every bit identification of eligible patients; data quality; unstructured data; interoperability; information sharing and patient privacy; data access and patient privacy; and human being resources.
Identifying Eligible Patients
Retrieval protocols and phenotyping methods are commonly practical against EHR data to define the denominator of interest and identify eligible patients for screening, clinical trials, and inclusion in registries.52 Computational phenotyping involves operationalizing process, consequence and example definitions as a prepare of measures that tin can be captured during regular episodes of clinical care and that are stored in the EHR. General categories of data that are drawn for computational phenotyping from EHRs include medications, laboratory tests, and diagnoses.52 Operationalized definitions can be used for a number of applications including cohort screening and identification to enable clinical research; assessments of electric current healthcare delivery processes and outcomes; and, changes due to new healthcare practices and interventions. Common for any of these applications is a need to evaluate the operational definitions that are used. Given that EHR data are collected for the purpose of documentation and are collected at various points in time for each patient, there are a number of opportunities for potential biases to arise and for data to exist missing. As such, a sound evaluation of the measurement approach is required prior to the employ of those measures for secondary analyses of cohort screening and identification. To appointment, there have been a meaning number of studies requiring cohort identification that report common measures such equally positive and negative predictive values, sensitivity, and specificity prior to conducting downstream analyses. The evaluation of measure out results depends in part on the intended apply of an operational definition and EHR data source(s). Some frameworks accept been adult to assist investigators in characterizing potential limitations to the use of operational definitions with EHR registry data and then that when analyses are performed the confidence level of those findings can exist quantified.52 , 65
Various challenges with denominator and variables selections exist when extracting data from EHRs for registries. Ambiguous phenotyping algorithms and lack of standardized retrieval protocols oftentimes result in selecting a denominator of patients from an EHR that is irrelevant, skewed, or biased for a registry. Multiple factors can be used to modify and refine the definition of a population denominator (eastward.g., age, gender, diagnoses, medications, lab results, radiologic findings, special atmospheric condition such as disability, and administrative information such as insurance coverage). Selecting the timeframes of the EHR data extract is also complex and may effect in incomplete temporal information represented in registries. Despite the higher interoperability of EHR information and standardization of phenotyping protocols, fine details of EHR data may affect the pick results. Some of the challenges include:
-
Process of Care: different providers or clinical workflows generate different data values for the same event or fact; hence, the same fact or event might be represented differently in the aforementioned EHR.
-
Nature of Intervention: dissimilar interventions with different levels of risk may be encoded similarly, significant EHR does not contain the true take a chance factors for those interventions.43 , 66
Data Quality
Equally a basic good practice, registries should utilize some grade of data curation to review and assess data quality. In the context of EHR-based registries, data quality issues stem from the fact that data extracted from EHRs oft requires extensive cleaning and grooming before existence imported into registries. EHRs are designed to manage the transaction of healthcare and support clinical workflow and documentation for billing. The purpose of an EHR is not to deport enquiry, and EHRs are non designed to systemically collect research-course longitudinal data. As a result, data captured by EHRs are of variable quality.fourteen , 45 For case, EHRs often house reliable laboratory and medication information for clinical purposes, simply EHRs typically lack consistent and sufficiently detailed information on risk factors, levels of education, or socioeconomic status.16 The quality of source data tin bear upon both the underlying data as represented in a registry and the results generated using such data. Thus, EHR data may not be appropriate for some research purposes.
Data quality can be defined in various perspectives. The most impactful aspects of data quality for registries are:14
-
Accuracy: the extent to which data captured in EHR accurately reflects the state of interest, which is often complex to measure because the truthful value of a given variable remains unknown.
-
Abyss: the level of missing data for a particular data element in the EHR for the population of interest; this is commonly measured as a data quality indicator for EHR-integrated registries. It is of import to annotation that for research purposes, a distinction is made between "must-have' and "overnice-to have" information, recognizing that completeness of "must-have" information is near important.
-
Timeliness: the length of fourth dimension between the initial capture of a value and the time the value becomes available in the EHR.
Information technology is important to notation that information quality varies beyond EHRs used by different healthcare organizations. Moreover, changes may be fabricated to EHR systems "behind the scenes" that affect information quality. For instance, upgrades intended to improve functioning or add features may inadvertently event in poor record linkage or may require updating record extraction protocols. Evaluating data quality, completeness and accuracy should be conducted as an on-going procedure and not a one-time exercise.
Unstructured Information
EHRs contain a considerable corporeality of unstructured data, such as progress notes. The loosely structured nature of typed text (also known as 'free text') is constructive in day-to-day clinical workflows but presents a major challenge for automating EHR-based registries. The unstructured data may contain key patient information missing in structured information, extra information complementing structured data, or fifty-fifty data that may contradict information represented past structured data. The complexities of unstructured data, forth with the fact that existing text mining tools and natural language processing applications have express accurateness in extracting information from free text,67 have prompted some registries to ask for a manual chart review of individual patients before concluding inclusion in the registry. Unstructured data limits the awarding of automated computational phenotyping methods and increases the likelihood of low data quality (e.g., missing information) when data are extracted from structured EHR data only.
Many EHRs also permit a choice of places where important information may be entered. For instance, some EHR take been set to facilitate quick entry of "easy treatments" that and then results in fragmented storage of treatment information. Treatment information may also be cached in clinical notes, which may non be accessible for research purposes since notes oft include a patient's name and other personally identifiable data that can be difficult to spot and redact systematically.
Interoperability
Interoperability is defined equally the ability of a system to exchange electronic health information with, and use electronic wellness information from other systems without special endeavour on the part of the user.68 Interoperability requires multiple stages, 'sending', 'receiving', 'finding' and somewhen 'using' the data.68 As discussed in Affiliate 1, interoperability spans multiple dimensions of standards: regulatory, contractual, privacy, substitution formats, content, and engineering science.68 , 69 In the context of EHRs and registries, syntactic interoperability is the ability of heterogeneous health information systems to exchange data with a registry, and semantic interoperability implies that the registry understands the data exchanged at the level of defined domain concepts.
From an EHR/registry perspective, functional interoperability could be described as a standards-based solution that achieves the following gear up of requirements: "The power of any EHR to exchange valid and useful information with any registry, on behalf of any willing provider, at whatever time, in a style that improves the efficiency of registry participation for the provider and the patient, and does not require significant customization to the EHR or the registry arrangement."3
Although interoperability of EHRs with other EHRs and wellness IT systems has increased over the last decade,70 virtually health systems do non share in-depth EHR-level data with other health systems. Lack of interoperability is a major limiting factor for the extraction, integration, and linkage of EHR data for registries. Most EHRs are not fully interoperable in the core functions that would enable them to participate in various registries without a significant endeavor.3 This deficiency is directly related to a combination of technical and economic barriers to EHRs' adoption and deployment of standards-based interoperability solutions.iii EHR vendors too provide heavily customized versions of their own systems for each client thus creating additional barriers to interoperability.3 Since registries seek data across large and generalizable populations, making EHRs interoperable across providers is a key stride in facilitating EHR-based registry efforts.
Information sharing and interoperability challenges are non limited to incoming EHR data for a registry. In a learning health system, a bidirectional registry shares its findings with providers that have shared their EHR data. In such a reciprocal model, the findings are turned into knowledge and can effectively be used to change the commitment of care and better outcomes across all participating providers. Currently, in that location are no common standards on how to distribute registry findings while protecting the identity of individual healthcare providers. Sharing the findings about data quality issues with information providers is challenging likewise as information technology may result in legal ramifications (eastward.yard., individual providers might become liable when information is captured inaccurately).
Linking and integrating various EHR information sources for registries also requires matching patients across databases. HIEs are sometimes required to generate primary patient indexes (MPIs) to lucifer patients across diverse EHR information sources. Developing and utilizing an MPI is a complex process and may introduce mistake and bias in registries despite many tools beingness available to reach this process.9 It is worth noting that most of the data elements needed to create MPI are considered protected health information co-ordinate to HIPAA regulations and may not be available for registries to complete the matching process.
EHR Infrastructure and Deployment
EHRs may provide IT infrastructure and tools to support the development of an EHR-based registry, but they typically do not provide turnkey solutions for functional registries. Over the last decade, a diverseness of EHR tools take been developed that could form the building blocks of EHR-based registries. For example, EHR-based clinical data warehouses collect and store EHR data across an entire health network. These system-wide data warehouses often serve as the courage of data products that eventually back up an EHR-integrated registry (run into Chapter 2). Withal, challenges with updating, maintaining, scaling, and sharing such tools across healthcare providers however hinders development of registries.
In addition, the architecture of an EHR deployment inside a healthcare delivery organization may influence the usefulness of EHR for different registry applications. For example, a wellness arrangement that lacks an enterprise-level EHR compages may find it challenging to develop a system-wide EHR-integrated registry, as each of its entities operates a standalone EHR with no interoperable solution to share data among them.
Data Access, Privacy, and Use
Data access and privacy challenges are circuitous in multi-site EHR-based registries. Chapters seven and 8 of the User's Guide provide more information on ethics, informed consent, and protecting patient privacy. Information sharing is an additional concern in the context of EHR-based registries. Decisions must be made virtually whether a single institutional review board (IRB) will suffice or whether all sites will require local IRB approval. Governance is also challenging as the rules around sharing of data (identifiable or de-identified) vary depending on the organizations involved and the purpose of the research.
Human Resources
Most healthcare providers, specially small function-based practices, do not take adequate staff time or even the necessary expertise to solve all potential challenges with EHR-registry integration/linkage. Indeed, several types of expertise are needed, such as:
-
Regulatory/ethics – what data can we share?
-
Scientific – what question is of import?
-
Research blueprint – how practise we answer the question?
-
Clinical – do the information mean what we think they mean?
-
Informatics – do the data maintain their epistemological integrity from clinical collection to analysis?
-
Information applied science (Information technology) – how do nosotros curate and manage the data?
-
Statistics and epidemiology – how do we answer the question with the data obtained?
In addition, although EHRs may offer cost-effective solutions for registry use, the need to capture comprehensive data for registries may counter this toll-effectiveness balance (due east.g., requiring costly changes to the clinical workflow). Assuming that all data objectives for a registry can be met inside an EHR, data collection for EHR-based registries hypothetically could be achieved at the time of a clinical encounter, thus reducing the cost of information drove; all the same, this has yet to be achieved on a widespread basis.
Other Factors
Other factors may also affect the usefulness of EHRs as a foundation for internal registries and/or for contributing to external registries. These include challenges with collecting patient consent within clinical workflows, incorporating patient-reported information, and safeguarding the security of the information.71
International Perspective on EHR-Based Registries
Some international registries are derived from national data nerveless in the context of national health insurance programs. In the Nordic countries, the unique constellation of universal coverage, a network of population-wide registries and databases, and individual-level linkage72 brand registries optimally suited for observational medical research in multiple clinical domains73 and, increasingly for pragmatic trials.74 – 76 In some countries, EHRs can be readily linked with the registry data using nationwide private identifiers. For instance, Nordic countries maintain a broad network of continuously updated databases, which collectively cover near health events, which can be linked on individual level in combinations dictated by the needs of a given study. In the United Kingdom, the Clinical Practice Research Datalink (CPRD)77 and The Health Comeback Network (Sparse) are important sources of routinely collected data, originating in EHRs. Both CPRD and THIN capture information routinely gathered in the form of daily operations of participating full general practices. The data undergo a set of congenital-in data checks before being available for research. In some instances, additional data are linked (east.g., hospital records, or basic socioeconomic data). All patients registered with the participating practices, regardless of their disease, are included in the resulting dataset as long as they are enrolled in a participating do.
Similarly, routine records are also being nerveless in some form in many countries in Europe though generally with less national coverage than in England, with not-exhaustive listing including Netherlands,78 , 79 Italy,79 , 80 Scotland,81 Frg,82 France,83 and Spain.84 In N America, routine health records from a single-payer organization are maintained by provinces in Canada;85 and, increasingly, in Asia, including South korea,86 and Taiwan.87 Although non originally established for inquiry, routine data have been playing an increasingly important role in studies of wellness and disease, including mail-marketing risk-management commitments.
The Futurity of EHR-Based Registries
The truthful promise of EHRs for registries is in facilitating the achievement of a applied, scalable, and efficient means of collecting registry data for multiple purposes. Scalability constraints on patient registries can be dramatically reduced by using digitized data.3 Paper records are inherently limited considering of the associated difficulty of systematically identifying eligible patients for inquiry activities and the attempt required to re-enter information into a database.3 Digitized information has the potential to make information technology easier to come across both of these requirements, enabling larger, more than diverse patient populations and avoiding duplication of try by participating clinicians and patients.3 However, duplication of effort can exist reduced but to the extent that EHRs capture data elements and outcomes with specific, consistent, and interoperable definitions — or that information can be found and transformed past other processes and technologies (e.g., natural language processing) into standardized formats that match registry specifications.3
Despite the challenges and barriers of using EHRs for registries, EHRs will likely play a fundamental office in expanding and developing existing and future registries. Multiple factors are poised to increase the office of EHRs in registries in the nearly future such equally:
-
increasing adoption of light-weight and efficient interoperability standards (east.chiliad., HL7 FHIR);88
-
new methods to mensurate EHR interoperability;69
-
innovative technical frameworks to harmonize the extraction of data from EHRs (due east.k., S&I Framework SDC);60
-
introduction of new EHR-embedded tools to develop EHR-integrated registries (e.g., ascertain and utilise retrieval protocols; additional EHR-integrated forms for registries);
-
incentivizing healthcare providers to share EHR data with registries (e.grand., Meaningful Utilise);89
-
adjustment value-based efforts and population health direction goals with reporting of EHR data to registries across providers (e.g., MACRA);90 and
-
providing additional clarifications nearly the application of HIPAA and other privacy protection rules in the context of EHR-based registries for both operational purposes and enquiry.91
EHRs tin can be linked or integrated with registries in many formats or various purposes. Futurity research should focus on developing and disseminating additional guidelines and technical documentations about registry integration with EHRs for public apply. Finally, achieving a fully interoperable EHR-based registry, so that EHRs and patient registries function seamlessly with one another, is unlikely to be accomplished in the near future.iii Withal, information technology is disquisitional that a level of interoperability be achieved to preclude the creation of information silos within proprietary informatics systems that make it hard or impossible to develop large EHR-based registries and conduct research across diverse practices and populations.
- c
-
EHRs are sometimes referred to as Electronic Medical Records (EMRs). This chapter uses both terms interchangeably.
References for Chapter 4
- i.
-
Gliklich R, Dreyer North, Leavy M, eds. Registries for Evaluating Patient Outcomes: A User'due south Guide. 3rd edition. Two volumes. (Prepared past the Outcome Determine Middle [Outcome Sciences, Inc., a Quintiles company] under Contract No. 290 2005 00351 TO7.) AHRQ Publication No. 13(14)-EHC111. Rockville, MD: Agency for Healthcare Research and Quality. April 2014. http://www
.effectivehealthcare.ahrq.gov. [PubMed: 24945055] - 2.
-
Aspden P, Corrigan JM, Wolcott J, et al. Key Capabilities of an Electronic Health Tape System: Letter Written report. Institute of Medicine of the National Academies; 2004.
- 3.
-
Gliklich RE, Dreyer NA, Leavy MB. Interfacing Registries With Electronic Health Records. Registries for Evaluating Patient Outcomes: A User's Guide. 2. Third ed. Rockville, MD: Bureau for Healthcare Research and Quality (AHRQ); 2014. p. iii–22. [PubMed: 24945055]
- 4.
-
Dixon BE, Gibson PJ, Grannis SJ. Estimating Increased Electronic Laboratory Reporting Volumes for Meaningful Employ: Implications for the Public Health Workforce. Online Journal of Public Wellness Computer science. 2014;5(three):225. PMID: 24678378. DOI: 10.5210/ojphi.v5i3.4939. [PMC complimentary commodity: PMC3959912] [PubMed: 24678378] [CrossRef]
- 5.
-
Electronic Health Record Incentive Program, 42 CFR 412, 413, 422, 495 (2010).
- six.
- seven.
- 8.
- ix.
- 10.
- xi.
-
Jha AK, Burke MF, DesRoches C, et al. Progress Toward Meaningful Utilize: Hospitals' Adoption of Electronic Health Records. Am J Manag Intendance. 2011;17(12 Spec No.):SP117–24. PMID: 22216770. [PubMed: 22216770]
- 12.
-
Centers for Medicare and Medicaid Services (CMS). Health Information technology: Standards, Implementation Specifications, and Certification Criteria for Electronic Health Record Technology, 2014 edition. Fed Regist. 2012 Sep 4; 77(171):54163–292. PMID: 22946139. [PubMed: 22946139]
- 13.
-
Centers for Medicare and Medicaid Services (CMS). Acute Care Hospital Inpatient Prospective Payment System. Medicare Learning Network. December 21, 2012. [PubMed: 22937544]
- 14.
-
Chan KS, Fowles JB, Weiner JP. Review: electronic health records and the reliability and validity of quality measures: a review of the literature. Med Intendance Res Rev. 2010;67(5):503–27. PMID: 20150441. DOI: 10.1177/1077558709359007. [PubMed: 20150441] [CrossRef]
- 15.
-
Madden JM, Lakoma MD, Rusinak D, et al. Missing clinical and behavioral health data in a large electronic health record (EHR) system. J Am Med Inform Assoc. 2016;23(6):1143–9. PMID: 27079506. DOI: 10.1093/jamia/ocw021. [PMC free article: PMC5070522] [PubMed: 27079506] [CrossRef]
- xvi.
-
Mendelsohn AB, Dreyer NA, Mattox Pow, et al. Characterization of Missing Data in Clinical Registry Studies. Therapeutic Innovation & Regulatory Science. 2015;49(1):146–54. PMID: 30222467. DOI: 10.1177/2168479014532259. [PubMed: 30222467] [CrossRef]
- 17.
-
Health Insurance Portability and Accountability Human action of 1996 (HIPAA), Pub. L. No. 104-191, 110 Stat. 139 (1996) (codification as amended in scattered sections of 42 UsaC.). HIPAA Privacy Dominion Regulations codification at 45 CFR pts. 160 & 164 (2010).
- 18.
-
(CDC) CfDCaP. International Classification of Diseases 10th Revision Clinical Modification (ICD-10-CM). https://www
.cdc.gov/nchs/icd/icd10cm.htm. Accessed August 16, 2019. - 19.
- twenty.
-
National Library of Medicine (NLM). SNOMED CT. 2017; https://world wide web
.snomed.org/. Accessed June 10, 2019. - 21.
-
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®): APA Publishing; 2013.
- 22.
- 23.
- 24.
-
George J, Phun YT, Bailey MJ, et al. Development and validation of the medication regimen complexity index. Ann Pharmacother. 2004;38(9):1369–76. PMID: 15266038. DOI: 10.1345/aph.1D479. [PubMed: 15266038] [CrossRef]
- 25.
- 26.
- 27.
-
World Health Organization (WHO). The Anatomical Therapeutic Chemic Nomenclature System with Defined Daily Doses (ATC/DDD). http://www
.who.int/classifications /atcddd/en/. Accessed Baronial 16, 2019. - 28.
- 29.
- 30.
- 31.
-
Townsend N, Rutter H, Foster C. Improvements in the data quality of a national BMI measuring programme. Int J Obes (Lond). 2015;39(9):1429–31. PMID: 25869597. DOI: x.1038/ijo.2015.53. [PubMed: 25869597] [CrossRef]
- 32.
-
National Institute of Health (NIH). Patient-reported Outcomes Measurement Data Organisation (PROMIS). http://www
.healthmeasures.cyberspace. Accessed Baronial xvi, 2019. - 33.
-
Committee on the Recommended Social and Behavioral Domains and Measures for Electronic Wellness Records; Board on Population Health and Public Health Exercise; Institute of Medicine. Capturing Social and Behavioral Domains and Measures in Electronic Wellness Records: Phase 2. Washington (DC): National Academies Printing (Usa); 2015 Jan 8. Abstract. https://www
.ncbi.nlm .nih.gov/books/NBK269341/. Accessed August xvi, 2019. [PubMed: 25590118] - 34.
-
Adler NE, Stead WW. Patients in context-EHR capture of social and behavioral determinants of health. N Engl J Med. 2015;372(8):698–701. PMID: 25693009. DOI: x.1056/NEJMp1413945. [PubMed: 25693009] [CrossRef]
- 35.
- 36.
-
Workman TA. Engaging Patients in Information Sharing and Data Collection: The Office of Patient-Powered Registries and Research Networks. AHRQ Community Forum White Paper. AHRQ Publication No. 13-EHC124-EF. Rockville, MD: Agency for Healthcare Research and Quality; September 2013. [PubMed: 24156118]
- 37.
- 38.
-
Arora S, Yttri J, Nilse W. Privacy and Security in Mobile Wellness (mHealth) Research. Alcohol Res. 2014;36(ane):143–51. PMID: 26259009. [PMC costless commodity: PMC4432854] [PubMed: 26259009]
- 39.
-
Dreyer NA, Blackburn S, Hliva V, et al. Balancing the Interests of Patient Information Protection and Medication Safety Monitoring in a Public-Private Partnership. JMIR Med Inform. 2015;3(2). PMID: 25881627. DOI: ten.2196/medinform.3937. [PMC free commodity: PMC4414957] [PubMed: 25881627] [CrossRef]
- 40.
-
Berwick DM, Nolan TW, Whittington J. The triple aim: care, wellness, and cost. Health Aff (Millwood). 2008;27(iii):759–69. PMID: 18474969. DOI: 10.1377/hlthaff.27.3.759. [PubMed: 18474969] [CrossRef]
- 41.
- 42.
-
Eggleston E, Klompas M. Rational Use of Electronic Health Records for Diabetes Population Management. Current Diabetes Reports. 2014;14(4):479. PMID: 24615333. DOI: 10.1007/s11892-014-0479-z. [PubMed: 24615333] [CrossRef]
- 43.
-
Duncan I. Healthcare Take a chance Aligning and Predictive Modeling. Winsted, CT: ACTEX Publications; 2011.
- 44.
-
Kharrazi H, Weiner JP. It-enabled Community Wellness Interventions: Challenges, Opportunities, and Future Directions. EGEMS (Wash DC). 2014;ii(3):1117. PMID: 25848627. DOI: ten.13063/2327-9214.1117. [PMC gratuitous article: PMC4371402] [PubMed: 25848627] [CrossRef]
- 45.
-
Kharrazi H, Lasser EC, Yasnoff WA, et al. A proposed national inquiry and development agenda for population health informatics: summary recommendations from a national expert workshop. J Am Med Inform Assoc. 2017;24(ane):two–12. PMID: 27018264. DOI: 10.1093/jamia/ocv210. [PMC costless commodity: PMC5201177] [PubMed: 27018264] [CrossRef]
- 46.
- 47.
- 48.
- 49.
- l.
- 51.
-
Observational Wellness Data Sciences and Informatics (OHDSI). https://www
.ohdsi.org/. Accessed June 10, 2019. - 52.
-
Hripcsak G, Albers DJ. Adjacent-generation phenotyping of electronic health records. J Am Med Inform Assoc. 2013;20(1):117–21. PMID: 22955496. DOI: 10.1136/amiajnl-2012-001145. [PMC free article: PMC3555337] [PubMed: 22955496] [CrossRef]
- 53.
-
Dreyer NA, Rodriguez AM. The fast route to evidence evolution for value in healthcare. Curr Med Res Opin. 2016;32(ten):1697–700. PMID: 27314301. DOI: ten.1080/03007995.2016.1203768. [PubMed: 27314301] [CrossRef]
- 54.
-
Arts DG, De Keizer NF, Scheffer GJ. Defining and improving data quality in medical registries: a literature review, case study, and generic framework. J Am Med Inform Assoc. 2002;9(half-dozen):600–11. PMID: 12386111. DOI: 10.1197/jamia.m1087. [PMC complimentary article: PMC349377] [PubMed: 12386111] [CrossRef]
- 55.
-
Modifications to the HIPAA Privacy, Security, Enforcement, and Alienation Notification Rules Nether the Health Information Technology for Economical and Clinical Health Act and the Genetic Information Nondiscrimination Act, 45 CFR Parts 160 and 164 (2013). [PubMed: 23476971]
- 56.
- 57.
-
Fiks A, Grundmeier R, Steffes J. Comparative Effectiveness Inquiry Through a Collaborative Electronic Reporting Consortium. Pediatrics. 2015;136(1):e215–24. PMID: 26101357. DOI: x.1542/peds.2015-0673. [PubMed: 26101357] [CrossRef]
- 58.
- 59.
- threescore.
- 61.
-
Lombardo JS, Burkom H, Pavlin J. ESSENCE II and the framework for evaluating syndromic surveillance systems. MMWR Suppl. 2004;53:159–65. PMID: 15714646. [PubMed: 15714646]
- 62.
- 63.
- 64.
- 65.
-
Pathak J, Kho AN, Denny JC. Electronic health records-driven phenotyping: challenges, recent advances, and perspectives. J Am Med Inform Assoc. 2013;xx(e2):e206–11. PMID: 24302669. DOI: x.1136/amiajnl-2013-002428. [PMC gratis article: PMC3861925] [PubMed: 24302669] [CrossRef]
- 66.
-
Richesson RL, Hammond Nosotros, Nahm M, et al. Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH Health Intendance Systems Collaboratory. J Am Med Inform Assoc. 2013;xx(e2):e226–31. PMID: 23956018. DOI: ten.1136/amiajnl-2013-001926. [PMC gratis article: PMC3861929] [PubMed: 23956018] [CrossRef]
- 67.
-
Ford East, Carroll JA, Smith HE, et al. Extracting data from the text of electronic medical records to improve case detection: a systematic review. J Am Med Inform Assoc. 2016;23(5):1007–15. PMID: 26911811. DOI: 10.1093/jamia/ocv180. [PMC costless commodity: PMC4997034] [PubMed: 26911811] [CrossRef]
- 68.
-
Benson T. Principles of Wellness Interoperability HL7 and SNOMED. Health Information science. 2010.
- 69.
- 70.
-
Fellow M, Charles D, Patel V, et al. Health Information Commutation Among U.S. Not-federal Acute Care Hospitals: 2008–2014. ONC Data Cursory. 2014(17).
- 71.
-
Kharrazi H, Chi W, Chang H-Y, et al. Comparison Population-based Risk-stratification Model Performance Using Demographic, Diagnosis and Medication Data Extracted From Outpatient Electronic Health Records Versus Administrative Claims. Medical Care. 2017;55(viii):789–96. PMID: 28598890. DOI: 10.1097/MLR.0000000000000754. [PubMed: 28598890] [CrossRef]
- 72.
-
Frank L. Epidemiology. When an Entire Land Is a Cohort. Science. 2000;287(5462):2398–ix. PMID: 10766613. DOI: ten.1126/science.287.5462.2398. [PubMed: 10766613] [CrossRef]
- 73.
-
Krueger WS, Anthony MS, Saltus CW, et al. Evaluating the Safety of Medication Exposures During Pregnancy: A Instance Study of Study Designs and Data Sources in Multiple Sclerosis. Drugs Real Globe Outcomes. 2017;iv(3):139–49. PMID: 28756575. DOI: ten.1007/s40801-017-0114-9. [PMC gratuitous article: PMC5567459] [PubMed: 28756575] [CrossRef]
- 74.
-
Raungaard B, Jensen LO, Tilsted HH, et al. Zotarolimus-eluting durable-polymer-coated stent versus a biolimus-eluting biodegradable-polymer-coated stent in unselected patients undergoing percutaneous coronary intervention (SORT OUT VI): a randomised not-inferiority trial. Lancet. 2015;385(9977):1527–35. PMID: 25601789. DOI: 10.1016/S0140-6736(14)61794-iii. [PubMed: 25601789] [CrossRef]
- 75.
-
Frobert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment tiptop myocardial infarction. Due north Engl J Med. 2013;369(17):1587–97. PMID: 23991656. DOI: 10.1056/NEJMoa1308789. [PubMed: 23991656] [CrossRef]
- 76.
-
Shurlock B. Randomization Within Quality Registries: A Toll-Constructive Complement to Classical Randomized Trials. European heart periodical. 2014;35(1):ane–two. PMID: 24382633. DOI: x.1093/eurheartj/eht493. [PubMed: 24382633] [CrossRef]
- 77.
-
Herrett E, Gallagher AM, Bhaskaran K, et al. Data Resource Profile: Clinical Practise Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827–36. PMID: 26050254. DOI: 10.1093/ije/dyv098. [PMC gratis article: PMC4521131] [PubMed: 26050254] [CrossRef]
- 78.
- 79.
-
Coloma PM, Schuemie MJ, Trifiro G, et al. Combining electronic healthcare databases in Europe to allow for big-scale drug safety monitoring: the EU-ADR Project. Pharmacoepidemiol Drug Saf. 2011;twenty(ane):1–11. PMID: 21182150. DOI: ten.1002/pds.2053. [PubMed: 21182150] [CrossRef]
- eighty.
-
Avillach P, Coloma PM, Gini R, et al. Harmonization process for the identification of medical events in viii European healthcare databases: the experience from the EU-ADR project. J Am Med Inform Assoc. 2013;20(ane):184–92. PMID: 22955495. DOI: x.1136/amiajnl-2012-000933. [PMC free commodity: PMC3555316] [PubMed: 22955495] [CrossRef]
- 81.
-
Alvarez-Madrazo S, McTaggart South, Nangle C, et al. Data Resource Contour: The Scottish National Prescribing Data System (PIS). Int J Epidemiol. 2016;45(3):714–5f. PMID: 27165758. DOI: ten.1093/ije/dyw060. [PMC free commodity: PMC5005947] [PubMed: 27165758] [CrossRef]
- 82.
- 83.
-
Boudemaghe T, Belhadj I. Information Resources Profile: The French National Compatible Hospital Discharge Data Ready Database (PMSI). Int J Epidemiol. 2017;46(ii):392-d. PMID: 28168290. DOI: ten.1093/ije/dyw359. [PubMed: 28168290] [CrossRef]
- 84.
-
Bolíbar B, Fina Avilés F, Morros R, et al. [SIDIAP Database: Electronic Clinical Records in Main Intendance equally a Source of Information for Epidemiologic Research]. Medicina Clínica. 2012;138(14):617–21. [PubMed: 22444996]
- 85.
-
Garies S, Birtwhistle R, Drummond Northward, et al. Data Resource Contour: National electronic medical tape data from the Canadian Primary Care Picket Surveillance Network (CPCSSN). Int J Epidemiol. 2017;46(4):1091–2f. PMID: 28338877. DOI: ten.1093/ije/dyw248. [PubMed: 28338877] [CrossRef]
- 86.
-
Cheol Seong S, Kim Y-Y, Khang Y-H, et al. Data Resource Contour: The National Health Information Database of the National Health Insurance Service in South korea. International Journal of Epidemiology. 2016;46(3):799–800. [PMC free article: PMC5837262] [PubMed: 27794523]
- 87.
-
Chen Y-C, Yeh H-Y, Wu J-C, et al. Taiwan's National Health Insurance Research Database: Authoritative Health Care Database as Report Object in Bibliometrics. Scientometrics. 2011;86(2):365–80.
- 88.
- 89.
- 90.
- 91.
How Do Pharmaceutical Companies Get Electronic Medical Data,
Source: https://www.ncbi.nlm.nih.gov/books/NBK551878/
Posted by: hadlockemenceapery2002.blogspot.com


0 Response to "How Do Pharmaceutical Companies Get Electronic Medical Data"
Post a Comment